Europe
RefluxStop™ is CE marked in Europe. It is currently available in:
- Germany
- United Kingdom
- Switzerland
- Spain
- Italy
- France
- Austria
- Sweden
- Norway
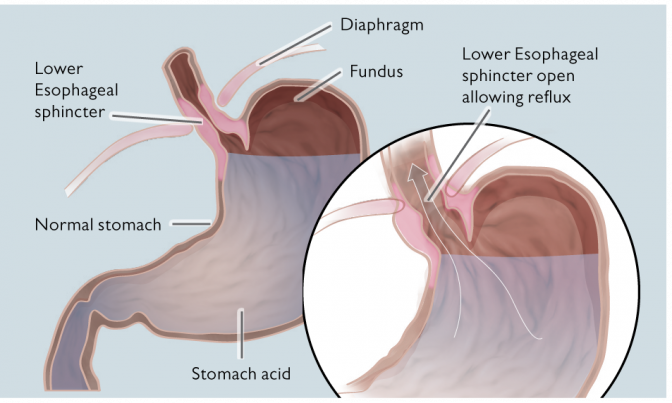
The RefluxStop™ procedure restores and maintains the position of the body’s natural Anti-Reflux Barrier (ARB), allowing its’ components to function normally.1
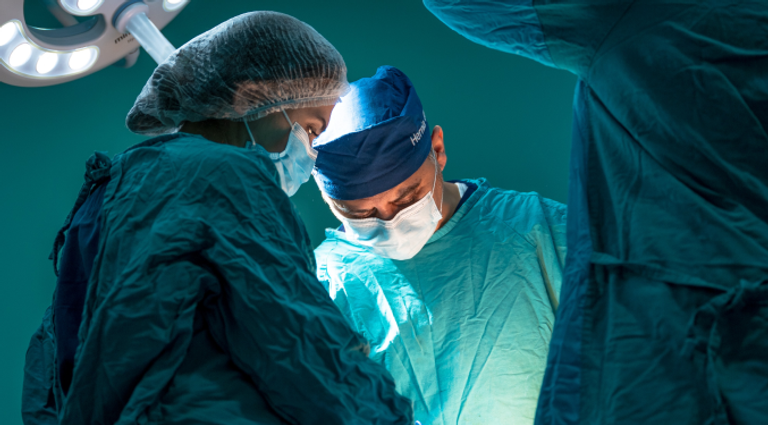
RefluxStop™ does not encircle the food passageway therefore unpleasant side effects associated with the standard of care are kept to a minimum.
By restoring all three components of the Anti-reflux Barrier (ARB)

RefluxStop™ is a medical device intended for patients 18 years and older. It was developed to treat acid and non-acid reflux in patients diagnosed with gastroesophageal reflux disease (GERD).
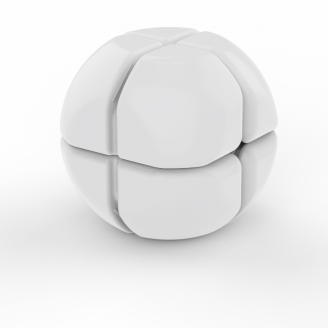
The sphere shaped device is made of medical grade silicone and is 24.5 mm in diameter, slightly larger than a Euro.
The RefluxStop™ procedure restores and maintains the position of the body’s natural Anti-Reflux Barrier (ARB) components, allowing it to function normally.

RefluxStop™ treats acid reflux without affecting the food passageway, an innovative method that will possibly create a paradigm shift in acid reflux treatment. This ingenious device is a non-active implant and is placed on the upper part of the stomach through laparoscopic (key hole) surgery. CE mark approval was granted August 8, 2018, on the strength of a multi-center clinical investigation in which the safety, effectiveness, and performance of the device in patients was demonstrated.
RefluxStop™ restores the body’s natural anatomy and physiology, treating acid reflux without encircling the food passageway.

Acid reflux is the second largest treatment field in the world, with over 1 billion sufferers globally1. RefluxStop™ treats the symptoms of acid reflux by eliminating the regurgitation of stomach fluid.
Acid reflux sufferers consumed drugs for USD 24 billion in 20102. We expect that the surgical treatment addressable market to grow substantially to reach a couple of million operations yearly3, and the clinical trial results support that RefluxStop™ has the potential to completely change the treatment of acid reflux.
We expect the addressable market to reach 2.4 million operations yearly3.

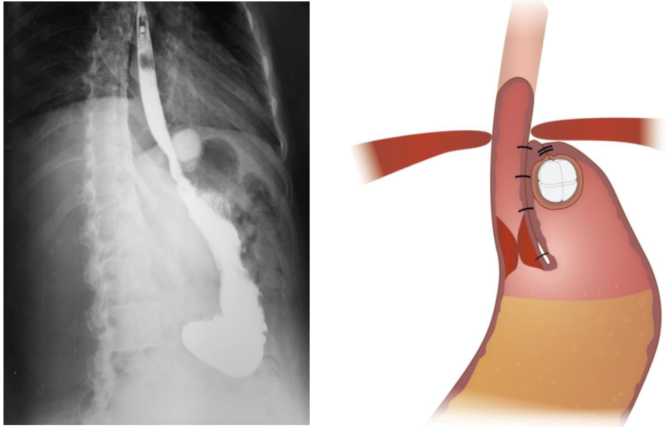
The device works with a proven concept to prevent the closing sphincter from gliding up into the thorax through the opening in the diaphragm breathing muscle. When this happens, the sphincter muscle enters the chest, and while breathing, it does not have enough power to close.
As per the design thesis’ double mechanism, RefluxStop™ device is intended to treat acid reflux without affecting the food passageway, including hindering the LES from entering the thorax.
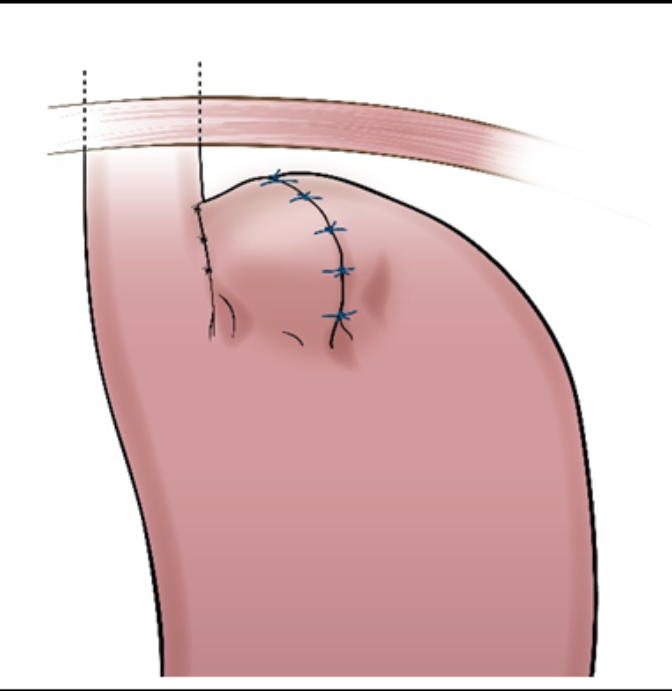
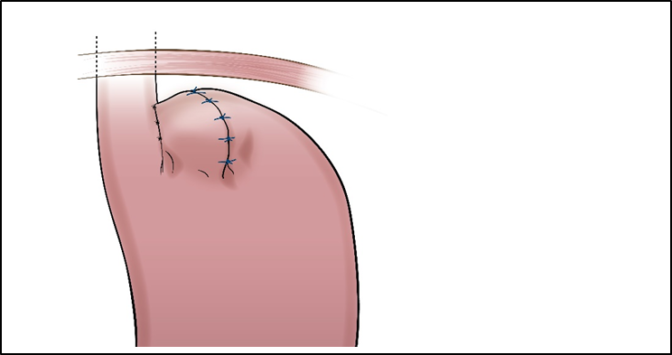
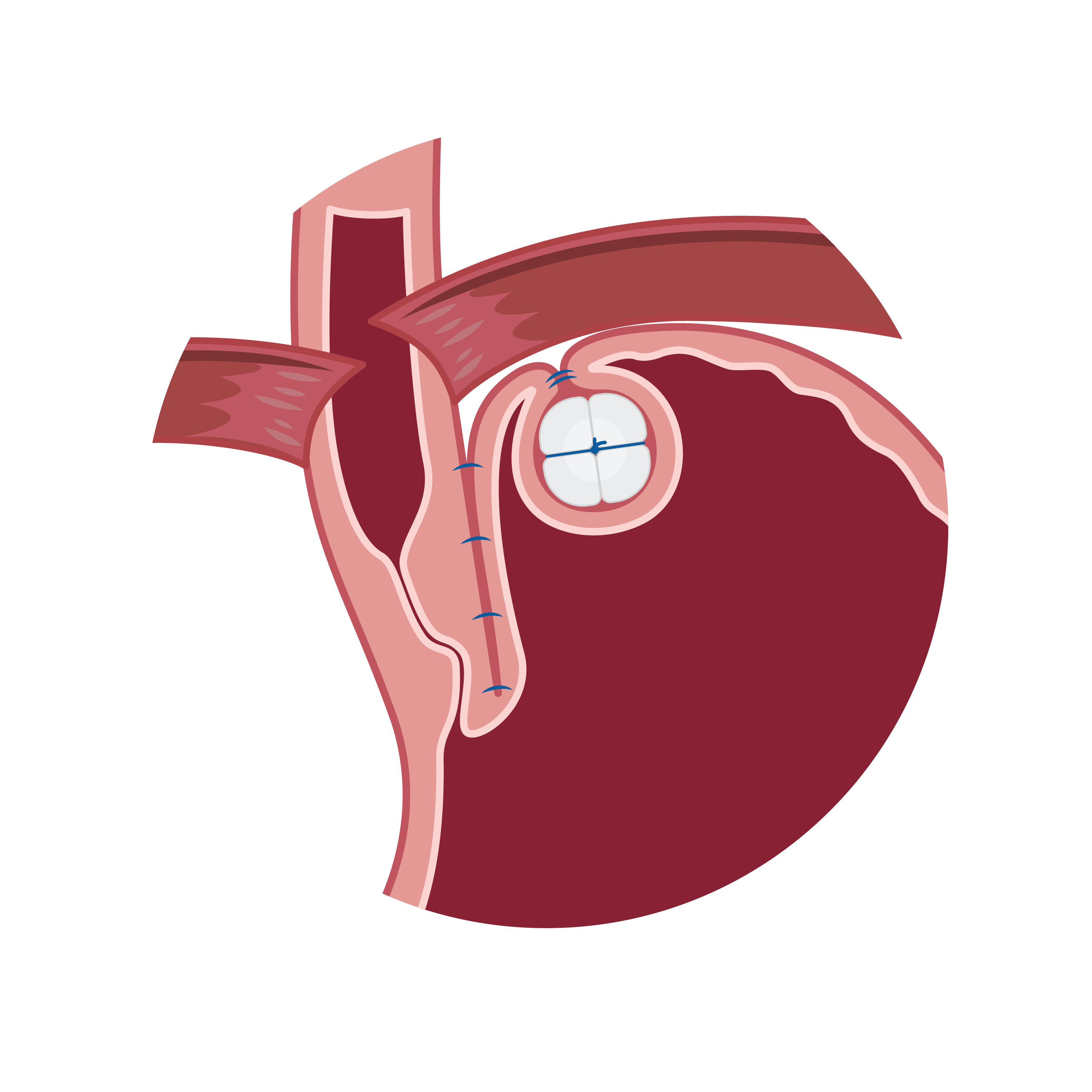
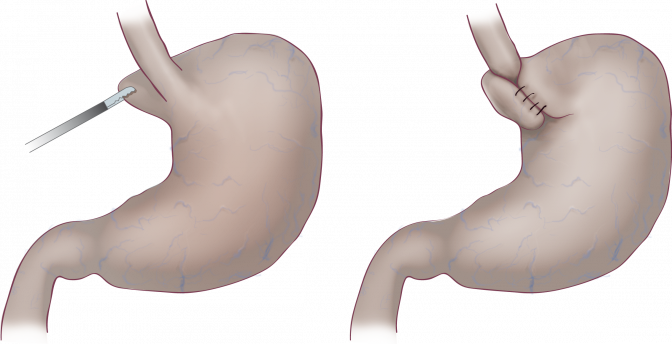
RefluxStop™ surgical procedure named the Forsell procedure after the inventor involves a reconstruction of the angle of His, a small left side adherence of the stomach to the esophagus, in broader terms also a fundoplication. RefluxStop™ device is invaginated in the fundus wall and thus reinforces the created cuff.
Sutures
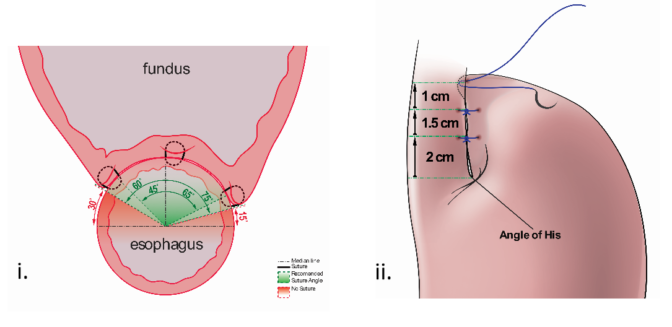
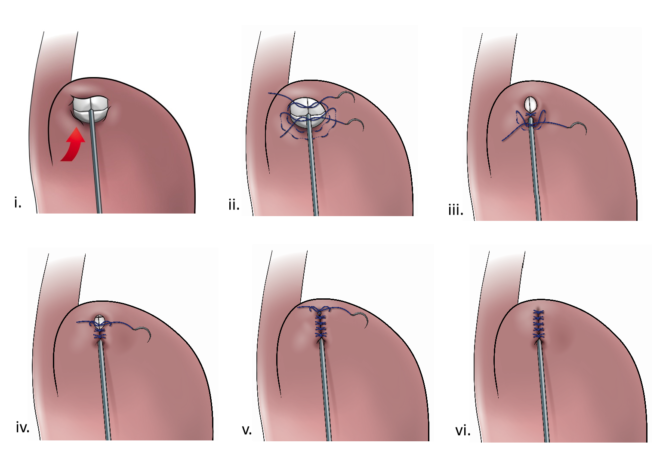
With RefluxStop™ device enclosed in the fundoplication cuff it becomes thicker/deeper, allowing the cuff to be created with less circumferential distribution, thereby reducing side effects related to the food passageway and at the same time treating GERD more efficiently.
Placing RefluxStop™ at fundus
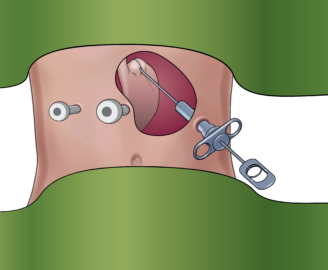
Removal of tool
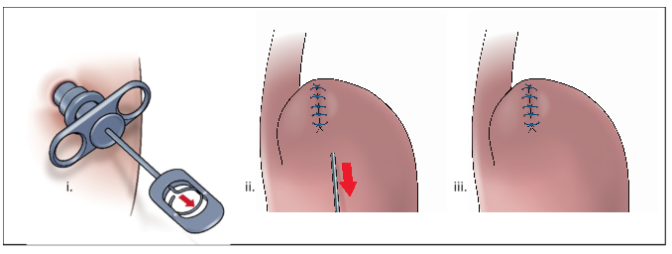
Finalized procedure
RefluxStop™ obtained CE mark certification on August 8, 2018. The approval enables Implantica to market and sell RefluxStop™ in member countries of the European Economic Area and Switzerland. Implantica, under confidentiality, informs about the clinical investigation month 6 results and the intended use of RefluxStop™.
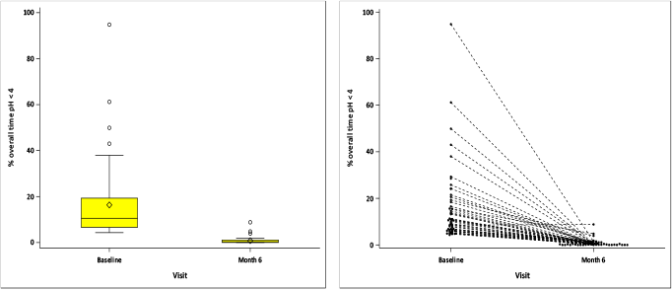
24-hour pH monitoring showed a 95% improvement after RefluxStop™ surgery, with the percentage of overall time with pH less than 4 substantially reduced to 0.8% at month 6, an excellent result. As soon as the results are published, they will be disclosed in more detail.
Using a validated questionnaire, GERD symptoms in total GERD-HRQL score were assessed before and 6 months after RefluxStop™ surgery.
Results at month 6-8 showed an average improvement of 91% in the total GERD-HRQL score with 97.9% having > 50% improvement (p-value<0.001). The total score dropped to 2.6 at month 6-8. One patient experienced less improvement because the device was placed too low at surgery.
No safety concerns were associated with the RefluxStop™ device at month 6. There were no deaths, no device deficiencies, device related complications (no ADEs, no SADEs), no device explantations and no complications (AEs) leading to withdrawal.
In comparison to a literature review and meta-analysis on Nissen fundoplication performed by the Karolinska Institute (978 articles reviewed – 50 randomised articles selected), the results of the RefluxStop™ clinical investigation show (95% confidence intervals):
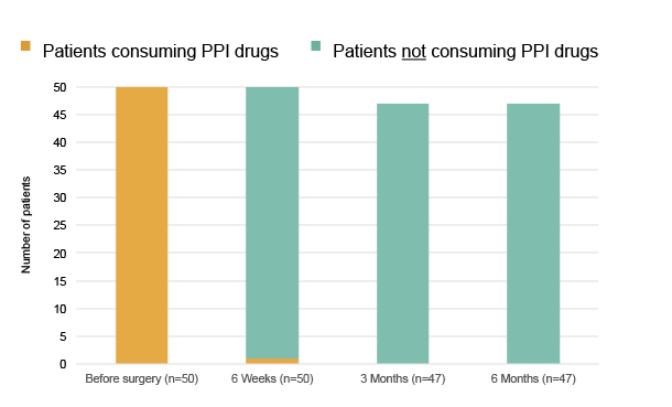
At month 6 after surgery all patients with evaluable data stopped taking PPIs.4 As soon as the results are published, they will be disclosed in more detail.
Acid reflux creates precancerous changes in the lower esophagus, called Barrett’s esophagus, by iterative damage caused by stomach fluid found in 10-15% of acid reflux patients4. For daily sufferers the incidence is even higher probably at 20%5. The most common treatment method, drug therapy, does not prevent acid reflux but only reduces the acidity in the regurgitating stomach fluid, and the majority of patients continue to experience heartburn now and then. 59% of drug users continue to intermittently have acid reflux6 and only 1 out of 9 become completely symptom free.6
The current standard of care surgical treatments of acid reflux often lead to complications, such as swallowing problems, because they encircle the food passageway. RefluxStop™ has a unique mechanism of action and does not encircle the food passageway thereby reducing complications, as supported by the clinical investigation.
The stages of progression of untreated GERD:

Disclaimer: There is no clinical data showing that RefluxStop™ can lower a patient’s risk of developing cancer.
RefluxStopTM successfully treats acid reflux by restoring and maintaining the body’s natural anatomy without encircling the food passageway, thereby minimizing common side effects commonly associated with standard of care acid reflux surgical procedures.
95% of patients did not need to take daily PPI medication
95.5% (42/44) of subjects had no or minimal/occasional episodes of regurgitation, and all (100%) subjects experienced improvement in regurgitation compared to baseline
GERD-HRQL score decreased by 90% from a baseline of 29.5 (24–33) to 3.0 (0–9.2) at 4-year follow-up
95.5% (42/44) of patients were satisfied at 4 years
93% of patients had resolution or improvement in gas-bloating
Acid reflux or gastroesophageal reflux disease (GERD) is the term describing a condition in which liquid, acid content of the stomach, regurgitates (or refluxes) up to the esophagus, the tube connecting the stomach with the throat. The condition is common and almost everyone experiences this from time to time. Acid reflux affects people of all ages and backgrounds. The condition turns into the chronic disease gastroesophageal reflux disease, commonly referred to as GERD, when the frequency of acid reflux is abnormal.
GERD is one of the most highly prevalent diseases in the world with 31% of people suffering on a weekly basis9 and around 400 million with daily problems10. GERD has a considerable financial impact on society11.
The most common symptom of GERD is heartburn, a burning pain from behind the sternum, the breastbone. Other symptoms in more advanced cases are chest pain, difficulty swallowing, pain when swallowing, coughing, wheezing and/or vomiting. Sleeping disorder often becomes part of these patients’ everyday life as lying down increases the risk of acids entering the esophagus. For all GERD patients, their quality of life is severely affected.
The most common way to treat GERD is through pharmaceutical treatment. Proton pump inhibitors, PPI drugs, are proven to be the most efficient non-surgical treatment for GERD, even though they only treat the symptoms and not the cause – reflux with lower acidity is still present. Also 59% of the drug users experience heartburn now and then.12
Since the 1950’s the standard surgical treatment of GERD has been the fundoplication procedure, even though side effects are common. This procedure is when the top part of the stomach is wrapped around the ring muscle closing between the stomach and esophagus, the lower esophageal sphincter (LES). Other surgeries, radiofrequency treatment, or several different endoscopic procedures have proven less effective and are not used much.
All of the fundoplication methods have one major drawback – they all compress the food passageway – thereby causing swallowing problems and the inability to belch and vomit. Implantica’s new device treats acid reflux without affecting the food passageway at all. The complications of existing surgical treatments are so frequent that only around 100’00014 operations are performed in the US and Europe p.a. – although the need easily could be seen as a multiple of tenfold.
The substantial size of this treatment field can be concluded from the annual drug consumption of USD 24 billion in 2010.15 Yet only 1 out of 9 drug consumers become completely symptom free, thus there is a large need for a complication-free surgical procedure.
The RefluxStop™ device is indicated for patients diagnosed with Gastroesophageal Reflux Disease (GERD) defined by abnormal impedance pH and/or pH testing.
Below listed diagnoses have limited RefluxStop™ experience and are considered to be more complex cases. In compliance with standard clinical procedure with similar types of anti-reflux surgeries, these patients should be handled with precaution when implanted with the RefluxStop™ device.
RefluxStop™ - Instructions for Use